
Myofascial Pain
(Trigger point referring to tooth # 21 replicating the pain complaint.)
- No sensory deficits noted in the neurosensory evaluation of the area around the implants or teeth.
- Tooth #21 not sensitive to percussion, palpation, thermal testing or ToothSlooth evaluation.
- Somatosensory blocking of the area did not relieve the pain.
- Pain was replicated by trigger point palpation of the superficial masseter muscle.
- Pain was relieved by triggerpoint injection into masseter muscle.
Myofascial pain is a controversial topic in the medical community. Due to the ecomony of Nature, one would expect that no matter what organ system is involved, basic pain mechanisms would be similar and involve similar neurotransmitters and receptors with some local variability. If this is the case, then myofascial pain should be explainable in terms of now accepted pathways for neuropathic and neurovascular pain. The neuroscience of myofascial pain is very sketchy and loosely defined. Efforts have been made to identify and characterize myofascial trigger points without clear and consistent results. Pain referral mechanisms have been proposed that suggest mechanisms not consistent with known or accepted neuropathological mechanisms. This is a major reason why the Travell concepts of myofascial pain are not widely accepted in medicine.
If we were to consider myofascial pain as a peripheral sensitization of nociceptors in muscle tissue, we could explain many of the characteristics of myofascial pain using known mechanisms of neuropathic pain. David Simons suggested a mechanism that came closer to the accepted pain mechanisms when he postulated that trigger points caused central sensitization. He suggested the following diagram in a 1994 paper:
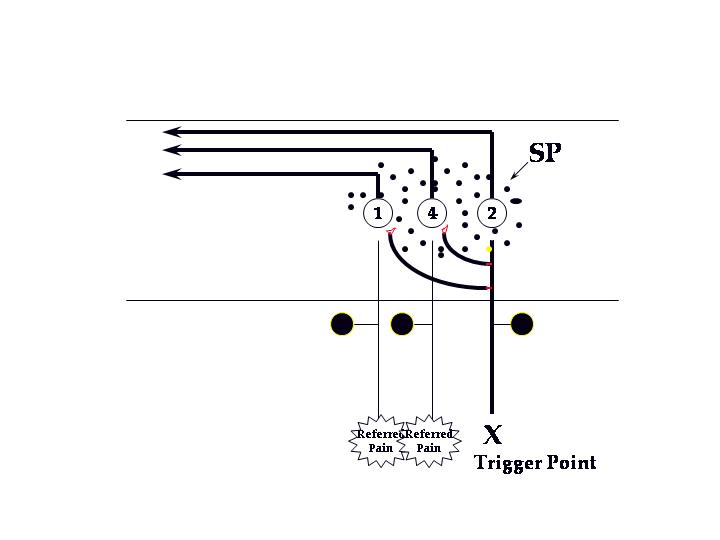
Current concepts of neurogenic and neuropathic pain suggest that pain fibers (small diameter unmyelinated nociceptors) release neurotransmitters both peripherally and centrally when activated. The mechanisms of peripheral sensitization are suggested in the following diagram:
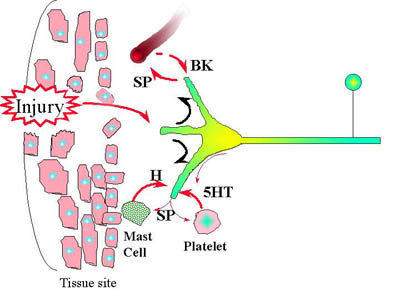
- localized swelling (taught bands and jump response?) in the muscle tissue
- spontaneous and palpable pain
- limitation of stretch due to pain and localized swelling.
- local response would lower the threshold to stimulation (pain with palpation)
- sensitization of adjacent peripheral nociceptor terminals, accounting, in part, for the expansion of the receptor field around the trigger point.
Peripheral sensitization leads to central sensitization. This is due to the ongoing central release of SP (as suggested by Simons), and Glutamate from the nociceptors. We now understand that glutamate activates the NMDA receptors on second order neurons in the TNC. When this occurs, there are a number of physiological responses that occur including further expansion of the peripheral receptive fields (referral site from the sensitized nociceptor?), lowered threshold to more pain, increased spontaneous activity, and prolonged afterdischarge, wind-up. Doesn't this sound like myofascial pain?
Questions to be answered by residents:
- In this scenario, why would a trigger point injection relieve the pain?
- Why would dry needling relieve pain?
- Why would stretching the muscle eliminate the trigger point and relieve pain?
- What is ischemic compression and why would it eliminate the trigger point and relieve pain?
Bibliography
- Simons, David G. Neurophysiological basis of pain caused by trigger points. Journal of the American Pain Society. 1994; 3(1):17-19.
- Simons, David G. Janet G. Travell Lois S. Simons. Travell & Simons’ myofascial pain and dysfunction : the trigger point manual. 2nd ed. Baltimore: Williams & Wilkins; 1999.
- Borg-Stein, J. and Simons, D. G. Focused review: myofascial pain. Arch Phys Med Rehabil. 2002 Mar; 83(3 Suppl 1):S40-7, S48-9.
- Hong, C. Z. and Simons, D. G. Pathophysiologic and electrophysiologic mechanisms of myofascial trigger points. Arch Phys Med Rehabil. 1998 Jul; 79(7):863-72.
Robert L. Merrill, DDS, MS
Director, Graduate Orofacial Pain Program
UCLA School of Dentistry